- Advice on the design of clinical trials, taking into account the different parameters required by the sponsor, researcher and / or the client; so that the study meets each of the methodological, statistical and regulatory requirements.
Our services
- Generation, adjustment and / or review of the necessary documentation for the approval and authorization of clinical studies by committees and regulatory entities.
- We have collaboration agreements with public and private institutions, as well as a wide network of referring physicians for the recruitment of study subjects.
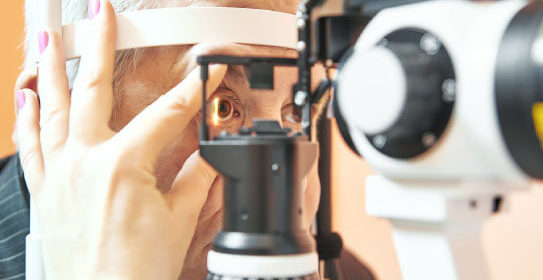
- We carry out clinical protocols for the study of drugs, biomedical and biological devices in the ophthalmology area, always safeguarding the integrity and rights of the patient, as well as their adherence to the clinical trial.
Clinical Research Unit
QUALITY POLITICS
Conducting clinical studies in ophthalmology with adherence to regulatory, ethical, and technological aspects, complying with the requirements of our clients and with the regulatory, quality, and safety standards of the organization’s own stakeholders.
CONDUCT OF CLINICAL PROTOCOLS
Our clinical research unit is certified in ISO 9001: 2015, comprising the processes of “Negotiation of the clinical study; Selection and recruitment of subjects for the execution of the protocol based on the requirements as a clinical research unit” that considers the context and the interested parties of the organization.
QUALITY OBJECTIVES
1. Recruitment: Comply with at least 95% of the number of participating individuals as voluntary subjects, in the time agreed with our clients.
2. Attachment to the patient: Maintain the percentage of patient attendance, in events that include the clinical study, greater than 90%.
3. Deviations: Have no more than 5% deviations from the protocol based on the requirements established by the client.
4. Protocol Recruitment Time: Reduce protocol recruitment time by 5% to that established by the client.